Let's take a look at the boilers formally. Here's the link to the previous article.
Previous Module
Definition of Boiler
A boiler can be defined as a closed pressure vessel where steam is generated by boiling distilled water under pressure. The heat for this purpose is obtained by the combustion of fuel in the boiler furnace. The hot gases so produced flow over the heating surfaces and transfer their heat energy to water.

Main Properties of Steam and Its Importance in Energy Transfer
Steam has many performance advantages that make it an indispensable means of delivering energy. These with respect to the other alternatives having advantages that include:
- Low cost (Water is a useful and cheap medium for transferring heat to a process.), low toxicity, clean, odorless, and tasteless.
- It can be available at any place, ease of transportability, flow rate can be easily controlled and readily distributed.
- High heat capacity (Steam holds a significant amount of energy on a unit mass basis, between 2325 and 2905 kW/Kg, that can be extracted as mechanical work through a turbine or as heat for process use).
- High efficiency (It is capable of supplying process heat at constant temperature while condensing; Since most of the heat content of steam is stored as latent heat, large quantities of heat can be transferred efficiently at a constant temperature, which is a useful attribute in many process heating applications).
- It can be used repeatedly again and again as well as first used for power generation and then for process heating.
- However caution is needed during phase change processes. When water is boiled into steam its volume increases about 1,600 times, producing a huge force. This causes the boiler to be extremely dangerous equipment that must be treated with utmost care.
Types of Steam
When water is heated to the boiling point and beyond, it changes into steam, or water in a gaseous state. The different combinations of pressure and temperature cause the properties of steam to vary widely.
Saturated Steam
Saturated steam is the most common type of steam. Steam in this state is constituted of both the liquid phase and gaseous phase of water. This means that the rate of evaporation is equal to the rate of condensation. Steam generated by a boiler is usually saturated steam. Saturated steam has properties which make it an excellent source of heat, and is hence widely used as a 100 °C to 200 °C heat source.
Wet Steam
This is the most common form of steam experienced by most plants. When steam is generated using a boiler, it usually contains wetness from non-vaporized water molecules that are carried over into the distributed steam. Even the best boilers may discharge steam containing 3% to 5% wetness. As the water approaches the saturation state and begins to vaporize, some water, usually in the form of mist or droplets, is entrained in the rising steam and distributed downstream. This is one of the key reasons why separation is used to disentrain condensate from distributed steam.
Dry Steam
Dry Steam is saturated steam that has been very slightly superheated. This is not sufficient to change its energy appreciably, but is a sufficient rise in temperature to avoid condensation problems, given the average loss in temperature across the steam supply circuit. Towards the end of the 19th century, when superheating was still a less-than-certain technology, such steam-drying gave the condensation-avoiding benefits of superheating without requiring the sophisticated boiler.
Superheated Steam
Superheated steam is created by further heating wet or saturated steam beyond the saturated steam point. This yields steam that has a higher temperature and lower density than saturated steam at the same pressure. Superheated steam is mainly used in propulsion/drive applications such as turbines, and is not typically used for heat transfer applications.
Supercritical Water
Supercritical water is water in a state that exceeds the critical point of water; 22.06 MPa, 373.95 °C. At the critical point, the latent heat of steam is zero. This implies that the specific volume of the part that is liquid is exactly the same as the specific volume of the part that is vapor. When water is hotter, or at higher pressure than critical point, it is in a state where water and steam are indistinguishable, a state that is neither liquid nor gas. Supercritical water is used to drive turbines in power plants which demand higher efficiency.
Marine Application of Boiler
Steam is used for the following auxiliary purposes on board:
- To heat HFO or FO in order to improve the viscosity for better atomization and to increase the transferability.
- Heating to separate impurities by centrifuging, settling, filtering etc
- Preheating the jacket cooling water of main engine for quick start and avoid thermal shock in cold climates
- Produce hot water and use in galley etc
- Operate deck machineries, feed pumps, cargo pump turbines
- Cargo heating (e.g. crude oil)
- Produce electricity by turbo generators
- Production of inert gas for cargo operation
The boiler used for this purpose is termed as an auxiliary boiler as the steam is not produced for the main propulsion of the ship.
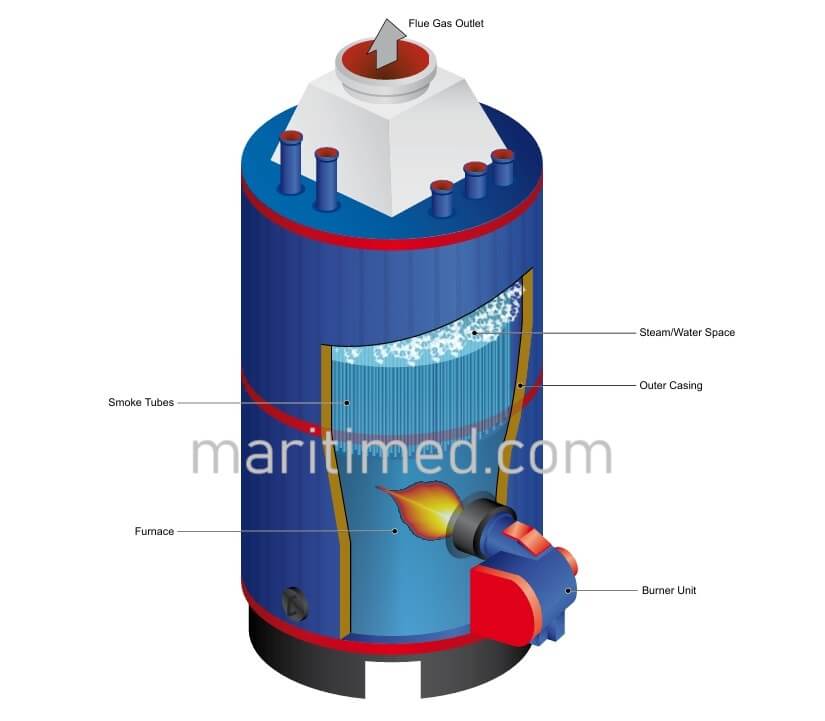
In case of a ship powered by steam, all the machineries, including the main propulsion machinery, are operated by steam. In such ships, more than two boilers can be installed. Earlier days steam engines were used where as now a days steam turbines are used for main propulsion e.g. in gas carriers etc.












