What if we tell you that you have already used boilers, if not used, then maybe observed someone using it before reading this article. Still thinking? Let's take our first dive into this topic with an understanding of the basics of this equipment.
Boiler- "Boil" er
As the name suggests, a boiler boils water into steam.
Before we go any further, why did we mention steam and not vapours? Let's clear it out.
Water Vapor
- Definition: Water in its gaseous state, formed when liquid water evaporates.
- Condition: Usually refers to an invisible gas at low pressure and temperature (like in the air we breathe).
- Example: The humidity in the air, or moisture from drying clothes.
Steam
- Definition: Water vapor that is produced by boiling water and typically at high temperature and pressure.
- Condition: It’s usually saturated or superheated, used for mechanical work or heat transfer.
- Example: The pressurized vapor coming from a pressure cooker.
| Feature | Water Vapor (Evaporation) | Steam (Boiling) |
|---|---|---|
| Definition | Gaseous H₂O formed by evaporation at any temperature | High-energy gaseous H₂O produced by boiling |
| Typical Temp / Pressure | Ambient temperature & pressure (e.g., room humidity) | Elevated temperature and pressure (e.g., 180 °C at 10 bar) |
| Visibility | Invisible until it condenses (fog / mist) | Often visible as a white cloud where it starts to condense |
| Energy Content | Lower — contains only latent heat of evaporation | Higher — latent plus sensible heat from higher temperature |
| Where It Forms | Surfaces exposed to air (oceans, puddles, wet laundry) | Inside boilers, kettles, turbines |
| Industrial Use | Rarely harnessed directly | Widely used for power, heating & mechanical work |
| Everyday Example | Humidity fogging a bathroom mirror | Jet from a kettle whistle or a ship’s boiler safety valve |
To summarize, Steam is the gaseous state of water that occurs when water is heated to its boiling point, while water vapor refers to water in its gaseous form at any temperature, including below boiling. Essentially, all steam is water vapor, but not all water vapor is steam, as steam is specifically associated with boiling conditions.
What is the difference between evaporation and boiling?
| Feature | Evaporation | Boiling |
|---|---|---|
| Definition | Slow change of liquid to gas at the surface | Rapid change of liquid to gas throughout the entire volume |
| Location | Only at the surface of the liquid | Occurs throughout the liquid |
| Temperature Required | Can happen at any temperature below boiling point | Happens only at the boiling point (e.g. 100°C at 1 atm) |
| Energy Source | May occur without direct heating (sun, ambient heat) | Requires a constant heat supply |
| Bubble Formation | No bubbles; molecules escape quietly | Bubbles form and rise as water changes into steam |
| Speed | Slow and gradual | Fast and vigorous |
| Everyday Example | Clothes drying, puddles disappearing | Water boiling on a stove, steam formation in a ship boiler |
| Relevance in Boilers | Not useful for energy generation | Primary method for creating high-pressure steam in boilers |
Remember, earlier we told you about an equipment which is a mini boiler in itself? Well, it's a pressure cooker. It is typically found in the kitchen of Indian households to cook food.
Boiler-Cooker Analogy
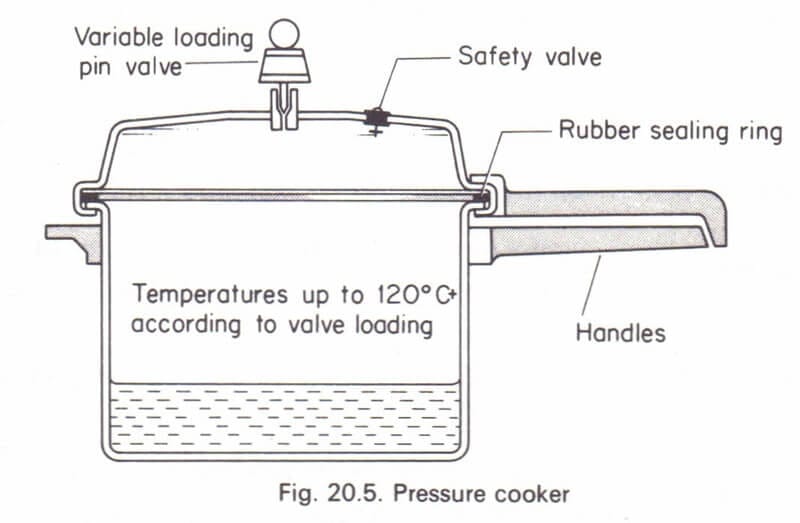
The water is stored in the cooker, and the lid is closed after ensuring the o-ring sealing ring is in place. The next step is to provide heat, after which the water inside the cooker starts boiling and steam formation takes place. To maintain a constant pressure in the cooker, a regulator is provided on the top. The weight of the regulator decides the pressure to be maintained in the cooker. When the pressure increases, the steam pressure exerted on the regulator becomes more than its self-weight acting downward, and it lifts to release the extra pressure. If, for some reason, the regulator malfunctions and the pressure keeps increasing, the cooker may overpressurize and burst. To save the scenario, a safety valve is provided to release the extra pressure.
On a similar note, we need to fill the boiler with water and provide it with the heat energy required to produce steam.
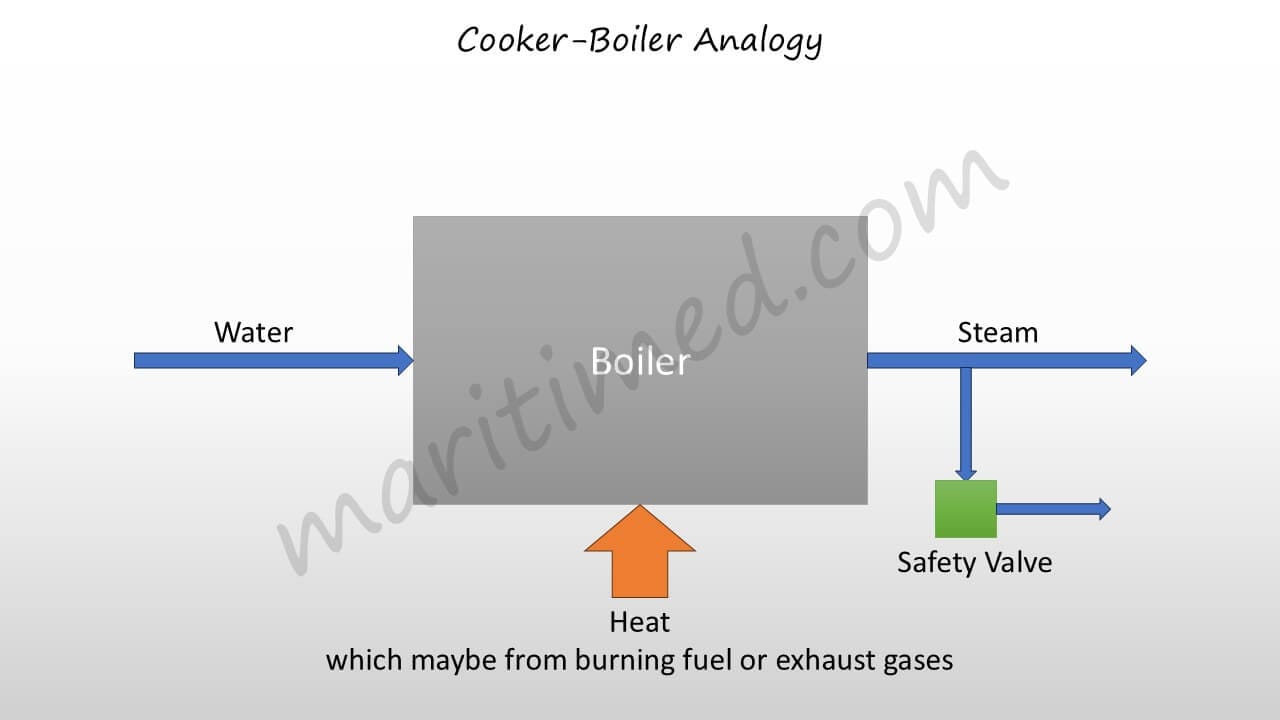
The boilers don't have regulators like the pressure cookers, which means the pressure regulation takes place in a different way. In case the steam pressure increases beyond the safe limit, the safety valve lifts to save the system. The steam produced is consumed by different systems either for heating applications, like heating up Fuel Oil, or to produce useful work, like producing power by turning steam turbines. We will discuss the above key points in greater detail in the upcoming modules.
Here's the next module, where we discuss the introduction in a more formal way.
Next Module












